Chemical Process Plant
Process Description:
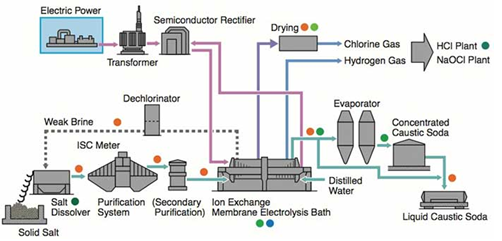
Chlorine – Caustic soda (sodium hydroxide or NaOH) is most commonly manufactured by the electrolysis of a sodium chloride (NaCl) solution using latest membrane technology. The principle is as follows:
Na+ + Cl- + H2O + electricity → ½ Cl2↑ + ½ H2↑ + NaOH
Sodium chloride solution (i.e. brine) is decomposed by electrolysis in electrolyzers to gaseous chlorine and to a sodium hydroxide solution (NaOH) at the anode of the cell and gaseous hydrogen at the cathode of the cell. The reaction of NaOH with Chlorine is prevented by a special membrane.
Caustic Soda
Product Description:
Caustic soda is a caustic metallic base, also known as Sodium Hydroxide (NaOH). It is a general utility chemical and constitutes one of the most important segments of the chemical process industry. It is produced (along with chlorine and hydrogen) via the chlor-alkali process. This involves the electrolysis of an aqueous solution of sodium chloride. The sodium hydroxide builds up at the cathode, where water is reduced to hydrogen gas and hydroxide ion. Pure caustic soda is a white solid; available in pellets, flakes, granules and as a 50% saturated solution. It is hygroscopic and readily absorbs water from the air.
Product Specification:
| CAS | 1310-73-2 |
| HS Code | 28152010 |
| NaOH | 99 percent min |
| Na2CO3 | 0.90 percent max |
| NaCl | 0.15 percent max |
| Fe2O3 | 0.007 percent max |
Product Application:
| Major Application | Usage |
|---|---|
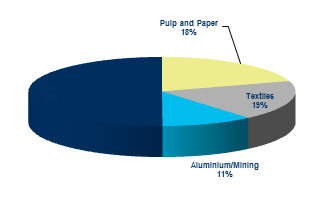
| Paper & Pulp | Caustic Soda is used as processing/ cooking agent of wood chips in the Kraft process. It also used for pulp bleaching and de-inking purpose. |
| Textile | Major application of Caustic is in the Viscose Rayon production. Apart from that it is used during mercerizing and scouring of cotton textiles and also during the dyeing operation of Nylon and Polyester fabrics |
| Aluminum | Caustic Soda is used for dissolving bauxite and separating alumina from the ore. Alumina is then purified to produce Aluminum in the Bayer process |
| Soaps & Detergents | Caustic soda is used for saponification of fats, tallow, and vegetable oil in soap and detergent manufacturing. When added in hot water, it is used as an industrial cleansing agent for stainless and glass bake ware, ovens, and industrial equipment. |
Hydrochloric Acid
Product Description:
Hydrochloric acid is the solution of hydrogen chloride (HCl) in water. It is a highly corrosive, strong mineral acid and has major industrial uses. It is found naturally in gastric acid. Hydrogen chloride is composed of diatomic molecules, each consisting of a hydrogen atom H and a chlorine atom Cl connected by a covalent single bond.
Product Specification:
| Product Name | Hydrochloric Acid |
|---|---|
| Appearance | Colorless, Fuming Liquid |
| Odor | Pungent Odor of Hydrogen Chloride |
| Solubiility | Infinite in water with slight evolution of heat |
| Density | 1.18 |
| pH | For HCL Solutions: 0.1 (1.0 N), 1.1 (0.1 N), 2.02 (0.01 N) |
| % Volatiles by volume @ 21C (70F) | 100 |
| Melting Point | -74C (-101F) |
| Vapor Pressure (mm Hg) | 190 @ 25C (77F) |
| Boiling Point | 53C (127F) Azeotrope (20.2%) boils at 109C (228F) |
Product Application:
| Major Application | Usage |
|---|---|
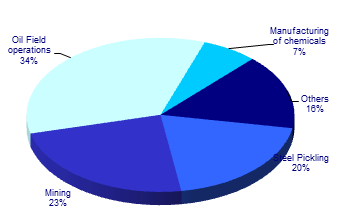
| Steel Pickling | Hydrochloric acid is used for pickling application wherein a dilute solution is used to remove the oxidized layer of chromium compounds in order to prevent rusting during rolling, unrolling etc. |
| Oilfields | Hydrochloric acid is used both to remove rust, scale and undesirable carbonate deposits in oil wells to encourage the flow of crude oil or gas to the well. It is also used to increase permeability of rocks by making them more porous. |
| Mining | Hydrochloric acid is consumed in many mining operations for ore treatment, extraction, separation, purification, and water treatment. Significant quantities are used in the recovery of molybdenum and gold. Hydrochloric acid is used to convert high-grade scheelite concentrate (CaWO4) and crude sodium tungstate to tungstic acid, which in turn, can be used to produce tungsten metal and chemicals. |
| Chemicals | Basic feedstock in the manufacture of a wide range of chemicals. It is used as an intermediate and reactant in processes that produce solvent, plastic, synthetic fibers, bleach, adhesives, herbicides, dyes, inks, and pharmaceuticals. |
Sodium Hypo Chlorite
Product Description:
Sodium hypochlorite is a strong bleaching chemical with a chemical formula NaClO. It is available in liquid as well as solid forms, and industrially manufactured demand is for 3-5%, 12% and 15% solutions. Chemically, it is possible to formulate as much as 40% concentration, but the commercial usage of such concentrations is limited.
Product Specification:
| Product Name | Sodium Hypochlorite |
|---|---|
| Appearance | Clear to yellowish liquid |
| Solubility | 100% |
| pH | 12-13 |
| % Volatiles by volume @ 21C (70F) | 100 |
| Melting Point | 25C |
| Vapor Density | 1.3 |
| Boiling Point | 101C |
Product Application:
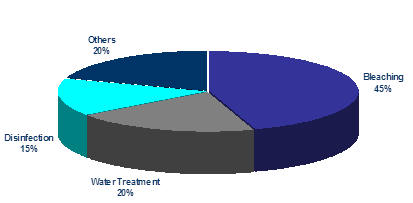
Sulphuric Acid

![]() Sulphuric Acid
Sulphuric Acid
![]() Linear Alkyl Benzene Sulphonic Acid (LABSA)
Linear Alkyl Benzene Sulphonic Acid (LABSA)
![]() Single Super Sulphate (SSP)
Single Super Sulphate (SSP)
![]() Stable Bleaching Powder
Stable Bleaching Powder
![]() Chlorinated Paraffin Wax (CPW)
Chlorinated Paraffin Wax (CPW)
![]() Hydrogen Peroxide
Hydrogen Peroxide
![]() Calcium Chloride
Calcium Chloride
![]() Ethyl Acetate
Ethyl Acetate
![]() Acetic Acid
Acetic Acid
